Reducing Risks of CMV Infection in Post-transplant Patients
Identify Patients at Risk of CMV Infection
Transplant patients are particularly susceptible to high morbidity and mortality from cytomegalovirus (CMV) infection due to suppression of cell-mediated immunity following transplantion.
With worldwide infection incidences of 60 to 80%, carefully monitoring post-transplant immune reconstitution of CMV-specific CD8+ T cells is essential for appropriate prophylactic or preemptive treatment1 while balancing the costs and potential toxicity (e.g., nephrotoxicity, peripheral blood cytopenia) associated with antiviral drugs.
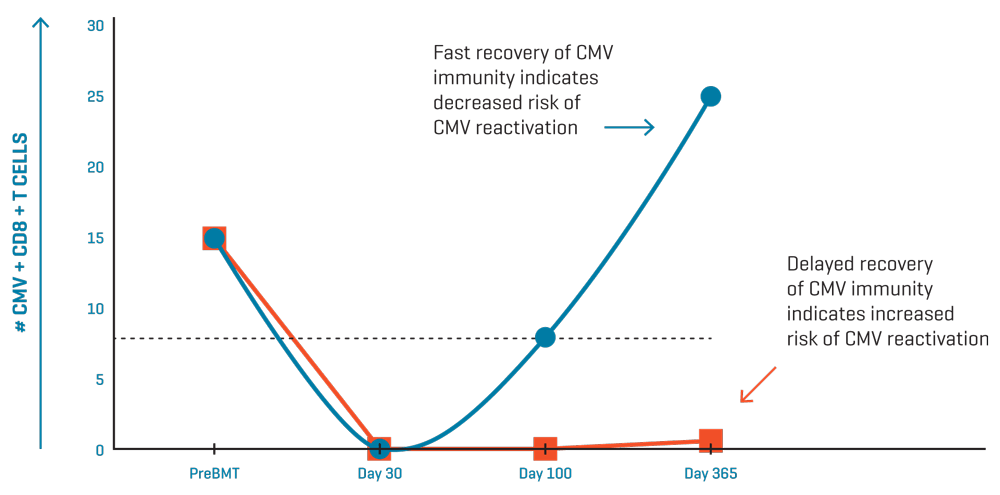
Model for CMV-specific T-cell immune monitoring in two patients post transplantation. Dashed line indicates threshold for recovery of CMV T cell immunity.
Refined CMV Management
Delayed recovery of CMV-specific T cells is associated with recurrent CMV infection and disease2-6. Several clinical trials have demonstrated the value of quantifying CMV-specific CD8+ T cells as a predictor of immune resistance to CMV after hematopoietic stem cell transplantation (HSCT).
Such a tool is especially useful to avoid unnecessary antiviral treatment of patients with a viral load who would never progress to CMV disease because they have sufficient T-cell immunity to effectively control the virus.
 Download the Flyer
Download the Flyer
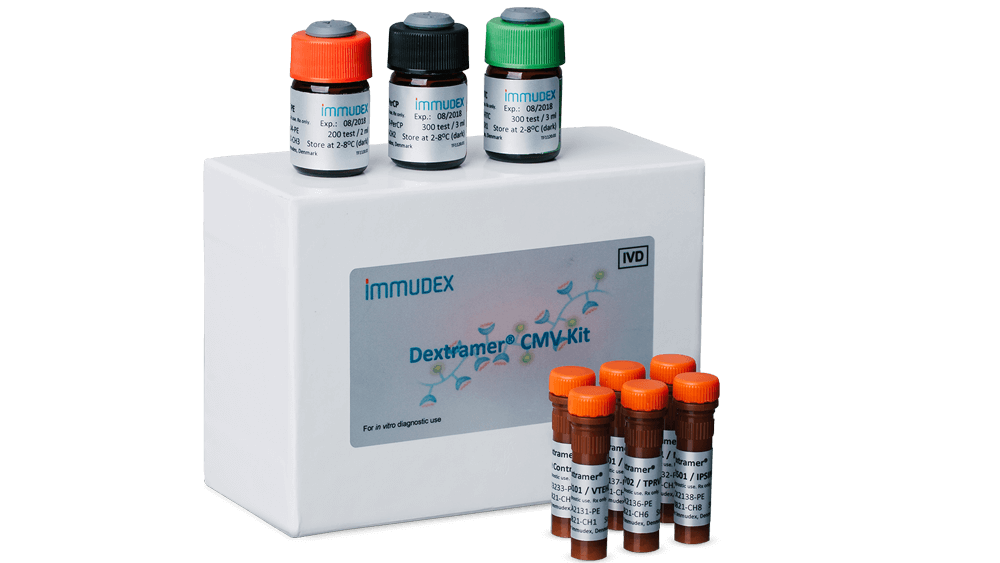
The Dextramer® CMV Kit (IVD) is designed to enumerate CMV-specific CD8+ T cells in whole blood by flow cytometry7. In fact, the Dextramer® CMV Kit (IVD) is indicated in conjunction with other laboratory and clinical findings to assess CMV-specific immune status and risk of CMV reactivation in adult HSCT recipients following immunosuppression8-9 .
The Dextramer® CMV Kit (IVD) cannot be used for measuring CMV infection or disease.
 Read the Study
Read the Study
Order Dextramer® CMV Kit (IVD)
Dextramer® CMV Kit (IVD) is available for in vitro diagnostic use in the EU and the US.
Order a kit by sending an email to [email protected] specifying the appropriate catalog number.
| Cat. No | Content | Regulatory Status | Package Insert |
|
CX02 |
CMV-specific Dextramer® reagents: Negative control Dextramer® reagent Antibodies: Anti-CD8, Anti-CD3, Anti-CD4 |
For in vitro diagnostics use in US 510(k) Premarket Notification: K153538 |
Link |
| CX03 |
CMV-specific Dextramer® reagents: Negative control Dextramer® reagent Antibodies: Anti-CD8, Anti-CD3, Anti-CD4 |
For in vitro diagnostics use in EU. IVDR 758042 |
Link |
Immudex has updated the catalog numbers. To learn more about it, please consult the document here
References
1. Badshah C. et al. . NEJM. 2017; 1-12. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation.
2. Cwynarski K, et al. Direct visualization of cytomegalovirus-specific T cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97(5):1232-40.
3. Gratama JW, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116(10):1655-62.
4. Gratama JW, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell depleted stem cell grafts and after transplantation may identify patients at risk of progressive CMV infection. Blood. 2001;98(5): 1358-64.
5. Borcher et al. Sequential anti-cytomegalovirus response monitoring may allow prediction of cytomegalovirus reactivation after allogeneic stem cell transplantation. PLoS One. 2012;7(12)
6. Tassi, E et al. Cytomegalovirus-specific T cells restricted for shared and donor human leukocyte antigens differentially impact on cytomegalovirus reactivation risk after allogeneic hematopoietic stem cell transplantation. Hemotologica . 2023 Jun 1;108(6):1530-1543. doi: 10.3324/haematol.2022.280685.
7. Tario J, et al. Dextramer Reagents are Effective Tools for Quantifying CMV Antigen-Specific T Cells from Peripheral Blood Samples. Cytometry B Clin Cytom. 2014;
88(1): 6–20.
8. TF1000.07 Dextramer CMV Kit Package Insert US (IVD), Immudex.
9. TF1293.01 Dextramer CMV Kit Package Insert EU (CE-IVD), Immudex.
