Get in Touch with our Immune Monitoring Experts
Analyze NY-ESO-1-specific T cell populations:
- Targeted detection: Choose MHC Dextramer® reagents from a range of HLA allotypes and a variety of different NY-ESO-1 epitopes.
- Exceptional avidity: Thanks to a flexible backbone, Dextramer® reagents bind even T cells with low affinity, which may go undetected with tetramers.
- Reliable results: High quality Dextramer® reagents ensure reliable experimental outcomes.
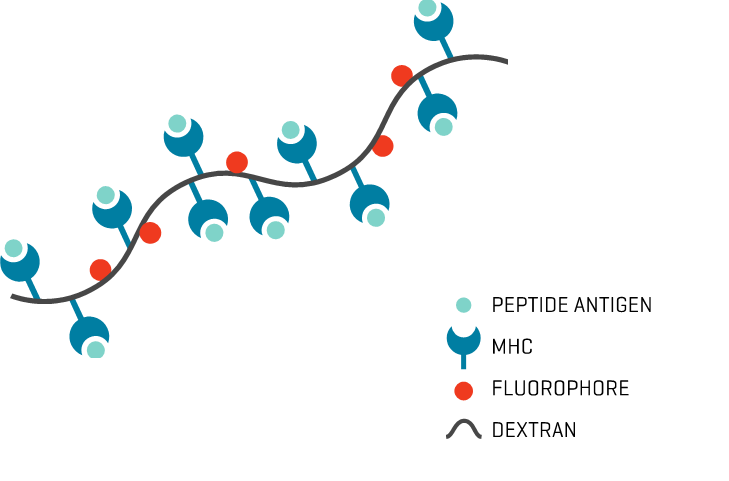
- Unambiguous signal: The multiple fluorophores per Dextramer® backbone enhance staining intensity for a clear signal and minimal background noise.
- GMP quality: MHC Dextramer® reagents are available in GMP quality, facilitating the translation of research findings into clinical reality.
NY-ESO-1 (New York esophageal squamous cell carcinoma 1), also known as LAGE2, is a cancer testis antigen expressed in various malignant cancers. Vaccines as well as TCR-engineered T cells are currently under investigation to target NY-ESO-1-expressing tumors. In fact, NY-ESO-1 was the most tested TCR target in a 2021 survey of cellular immunotherapies.
An essential aspect of validating a vaccine includes probing endogenous immune cell populations for NY-ESO-1-specific T cells. T cell therapy developers must also monitor the dynamics and persistence of transduced NY-ESO-1-specific T cells. Both demand highly granular examination of T cell populations.
MHC Dextramer® reagents are multimers designed for that purpose. Loaded with one or more epitopes, they deliver sensitive identification and specific discrimination between population subsets for a more complete picture of T cell responses.
 Reliable Detection of NY-ESO-1 Antigen-Specific T Cells with MHC Dextramer®
Reliable Detection of NY-ESO-1 Antigen-Specific T Cells with MHC Dextramer®
= orange
 Discover NY-ESO-1 TCR Solutions
Discover NY-ESO-1 TCR Solutions
"A thorough analysis of antigen-specific responses in T cells is critical to demonstrate efficacy of a T cell-based therapy and to investigate possible side effects."
- Thomas Holberg Blicher, PhD. Senior Research Scientist, Immudex
"The FDA recommended that we use a GMP-grade multimer for the purposes of lot-release testing our TCR-T based therapy."
- Scientist, Pharmaceutical Company

