Get in Touch with our Immune Monitoring Experts
Establish quality with Dextramer®
- Unmatched sensitivity: Dextramer® reagents sensitively and specifically bind engineered cells to accurately measure the transduction efficiency of your T-cell product.
- Highest quality: Our robust manufacturing according to GMP and quality control checks deliver MHC multimers meeting quality system requirements for medical devices defined by ISO 13485 and 21 CFR 820.
- Expert support: Whatever your specificity, phenotyping, or release testing requirements, our immune monitoring experts are standing by.
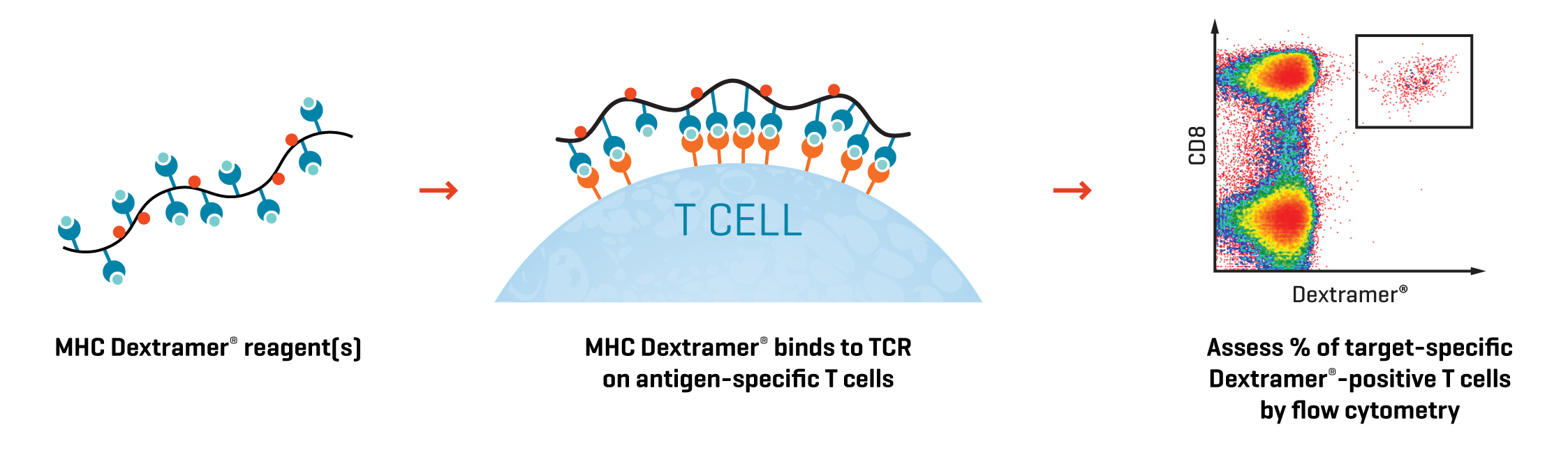
Release testing of T cell therapies is a rigorous battery of tests for sterility, absence of endotoxins and mycoplasma, and multiparametric flow cytometry to confirm T-cell identity, specificity, and percentage. Accurately counting antigen-specific T cells is essential and requires highly sensitive detection tools.
The architecture of Dextramer® makes it a fit-for-purpose reagent. The flexible molecular backbone affords high-avidity to detect and document the preinfusion phenotype of engineered cells, even with low transduction efficiency. The molecule carries multiple fluorophores to generate unambiguous detection signals making product consistency assessments easier. Finally, our Dextramer® GMP reagents meet stringent regulatory requirements.
 Learn more about Lot-Release Testing of TCR-T Therapies with Dextramer®
Learn more about Lot-Release Testing of TCR-T Therapies with Dextramer®
"The FDA recommended that we use a GMP-grade multimer for the purposes of lot-release testing our TCR-T based therapy."
- Scientist, Pharmaceutical Company
"Immatics uses Dextramer® reagents from Immudex for lot-release testing of TCR-based cell therapy product candidates. Being able to source high-quality pMHC multimer reagents from a reliable provider is important to us.”
- Kerry Sieger, M.A., VP of Global Quality Operations, Immatics

