- CAR detection reagents must adapt to the highly diverse body of CAR-engineered cell therapies in development pipelines.
- Unlike other commercially available reagents, antigen-based CAR detection reagents confirm not only the presence of the CAR on the surface of transduced cells but also their binding of the target antigen.
- Antigen-based CAR detection reagents have several advantages and cover various targets and labels. However, antigen multimers, like CAR Dextramer® reagents, also have the advantage of heightened sensitivity through multivalency.
CAR-T Staining Reagents
Avidity Can Make All the Difference in Detecting CAR-engineered Cells
The market approval of Kymriah® in 2017 marks the beginning of a new era in biopharmaceutical development characterized by the transition of living cells from producing to being active therapeutic agents. In the last years, hundreds of patients have been treated with bioengineered cells.
Today, the U.S. Food & Drug Administration has approved six commercial CAR-T cell therapies to treat seven hematological cancers¹ and one TCR-T cell therapy to treat advanced synovial sarcoma². And those numbers are likely to increase. As of March 2024, there were over 5000 clinical trials testing cell therapies on record. CAR-based therapies (using T cells or NK cells) accounted for the majority.³
There is an important distinction between the handful of approved CAR-T cell therapies and the plethora making its way along the path from bench to bedside. The approved therapies are conspicuously homogenous: four target CD19 using the FMC63 scFv, and the other two target BCMA. All use CD3ζ as signal 1 and either 4-1BB or CD28 as the costimulatory signal 2.
In contrast, the upcoming innovations in CAR-based therapies explore an expanded range of targets and a wide variety of structures and signaling strategies.⁴ That distinction raises questions about the adequacy of current tools to characterize the onslaught of diverse therapy candidates, not the least of which are staining reagents.
Beyond CD19 and BCMA: other targets that are being explored as targets for CAR-based products³
|
CD22 |
MSLN |
CD7 |
CD33 |
HER2 |
|
GPC3 |
CLDN18 |
B7H3 |
NKG2D |
CD70 |
|
GPRC5D |
IL15(R) |
NY-ESO-1 |
CD20 |
GD2 |
In this blog, we compare and contrast the different protein-staining reagent options that are commercially available. For a subset of those reagents that build on the target antigens of CAR constructs, we present and discuss performance data collected by our collaborator BioNTech SE in our blog post “The proof is in the staining: a comparison of antigen-based CAR detection reagents.”
What Are the Options Available for Detection of CAR-engineered Cells?
Say you’re developing a novel CAR-based cell therapy. How do you go about validating the functional expression of your CAR in lymphocytes, characterizing the transduced cell population, or monitoring CAR-engineered cell populations in blood over time? You could do it by measuring gene expression. However, to demonstrate the presence of the CAR on the cell surface, you will need protein staining reagents. Figure 1 summarizes different types of protein staining reagents currently used to analyze CAR-engineered cells.
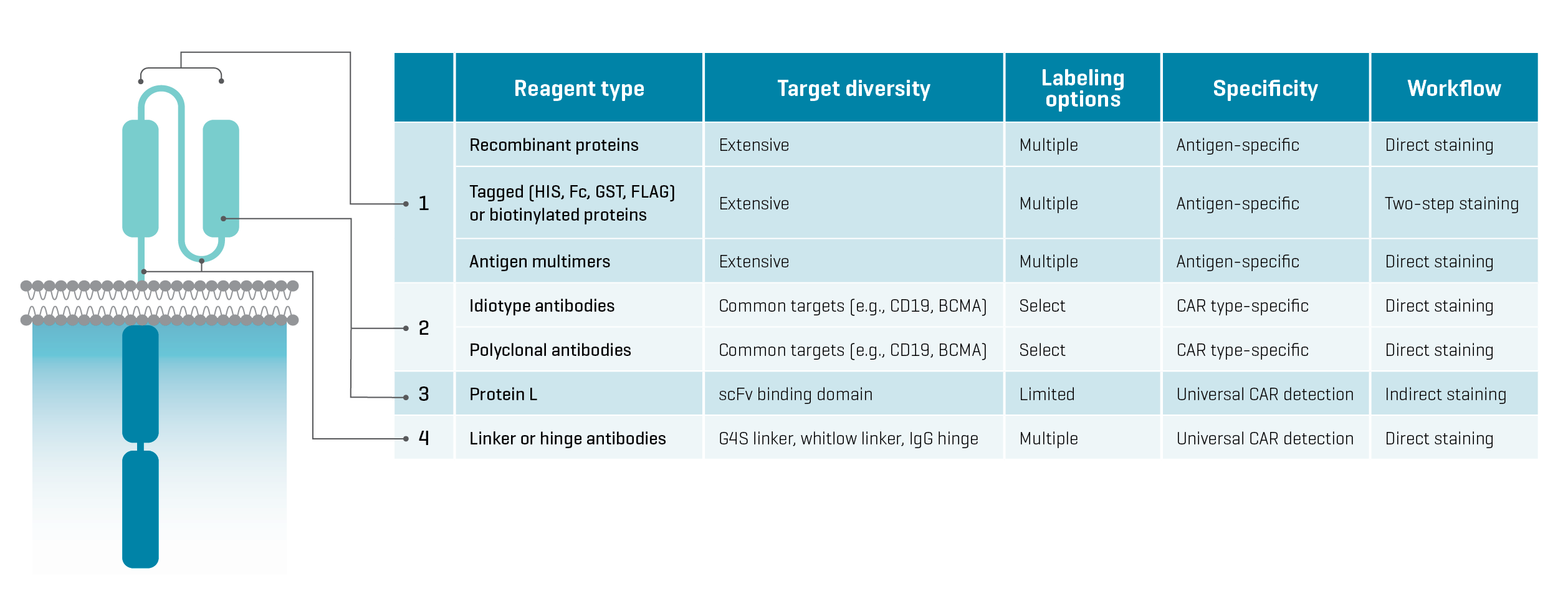
Figure 1. Features of protein staining agents currently used to characterize CAR-engineered cells. Binding locations on the extracellular domain of a CAR are labeled in the diagram on the left.
Commercial options for protein staining are plentiful. However, each type of detection reagent has features that can narrow down your selection.
Protein L, as well as linker and hinge antibodies, universally detect all CAR constructs that include defined protein sequences. They are agnostic to the CAR target. Direct staining is an option with all three reagents, which makes them easy to use. However, the utility of Protein L is constrained by its low sensitivity and incompatibility with human antibodies. Linker and hinge antibodies are also limiting because they can only be used with CAR constructs that include specific linker or hinge regions.
Idiotype and polyclonal antibodies are stable and versatile reagents purchasable from several commercial providers. They are specific to construct regions involved in target binding and thus, detect specific CAR types. However, commercial reagents offer only the most common CAR targets, like CD19, BCMA, CD20, and GD2. Detecting CARs with new targets would require developing dedicated antibodies at significant cost.⁵ Some polyclonal antibodies are also incompatible with blocking reagents and other antibodies used to phenotype cells, which can lead to inconsistent performance.⁶
Antigen-based reagents include recombinant proteins combining an antigen with a fluorophore label or a protein tag (e.g., HIS, Fc, GST) for direct or indirect staining, as well as antigen multimers. These reagents are specific to the antigen targeted by a CAR construct- this means that the detection of the CAR is based on the binding of its target antigen, demonstrating the CAR’s presence on the cell surface as well as its functionality.
Importantly, antigen-based reagents detect all CARs targeting a given antigen, regardless of construct architecture. This feature is a decided advantage in examining novel CAR constructs, including next-generation bivalent and other gated CAR strategies where it is important to demonstrate the concurrent expression of two extracellular domains. Antigen-based reagents are accessible and compatible with multiplex antibody staining, and commercially available options cover a broad spectrum of targets and labels, which affords workflow flexibility.
From a regulatory perspective, FDA Guidance for Industry recommends directly detecting the CAR to determine the percentage of CAR-positive cells. Antigen-based reagents meet this criterion. Furthermore, because they bind to the CAR construct in the same way that the target antigen would, their detection mode affords additional information about the functionality of the tested cell product.
FDA recommends direct detection of the CAR
FDA Guidance for Industry recommends the direct detection of CAR-positive cells. CAR Dextramer® reagents are a sensitive solution for direct detection and quantification of CAR-positive cells by flow cytometry. Read FDA Guidance
The Power of High-Avidity Multimers
Where recombinant and tagged proteins can fall short is in sensitivity, as they have a single valency for binding. Multivalent interactions with CAR constructs on an effector immune cell decrease their dissociation rate, resulting in a longer residence time on the cell (fig. 2). Hu et al. examined the sensitivity of monomer antigen-Fc chimeric proteins in detecting three different CAR constructs compared to multimer reagents. Titrations showed that the multimer reagents had significantly higher sensitivity, most dramatically for CAR constructs with high dissociation rates for their antigen ligand.⁶
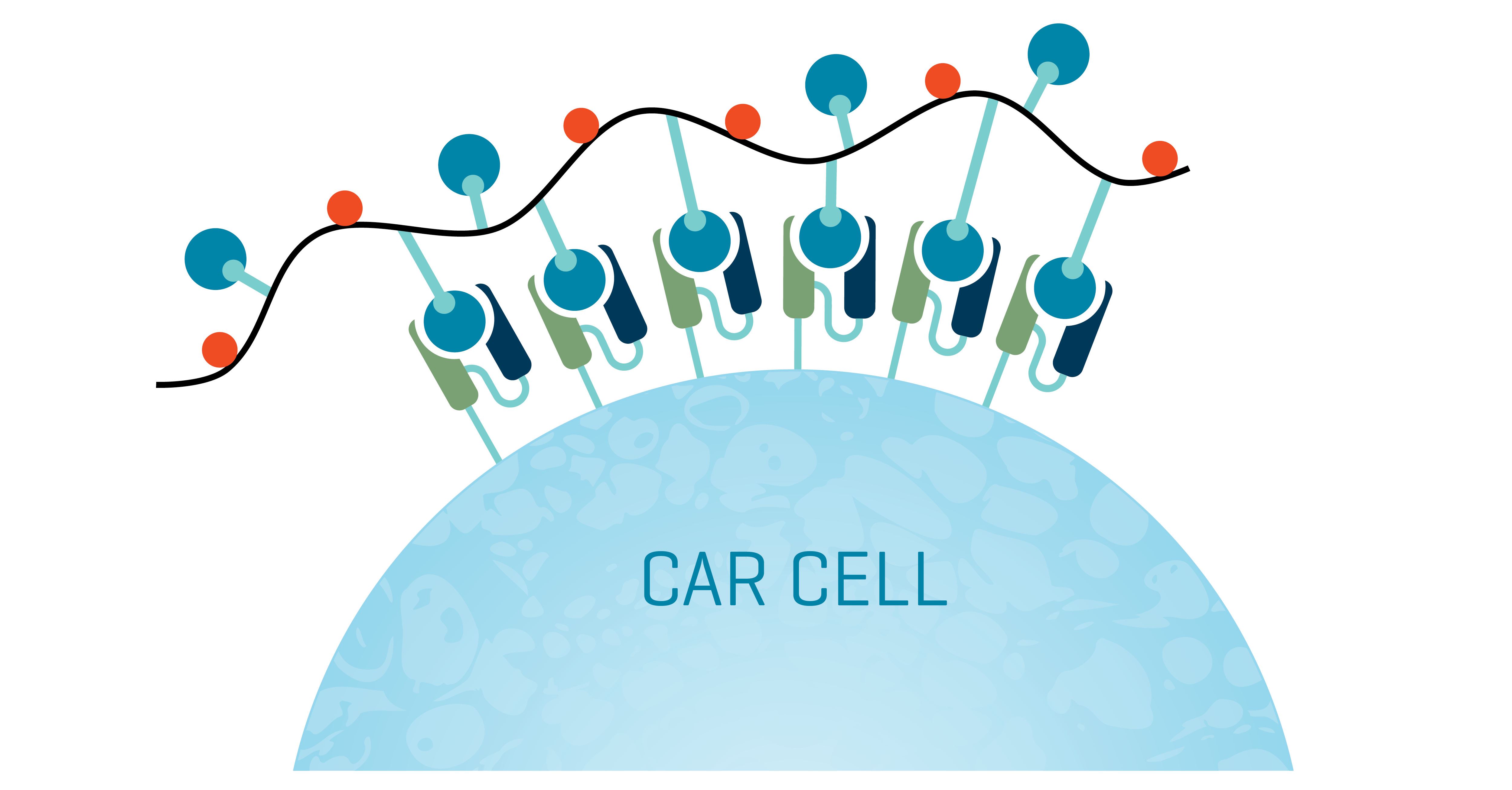
Figure 2. CAR Dextramer® reagents multivalently bind to CAR constructs on a cell. The resulting detection is sensitive and direct – a powerful solution to characterize CAR-positive cells by flow cytometry via a rapid one-step protocol.
High-avidity Antigen Multimers using Dextramer® Technology
With their dextran backbone displaying multiple binding sites, Immudex reagents magnify the antigen-specific detection of recombinant and tagged protein reagents with the high avidity of multivalent binding. Researchers worldwide use MHC I Dextramer® and MHC II Dextramer® reagents because they detect low-affinity antigen-specific T cells that are invisible to corresponding tetramers.
The same Dextramer® technology can be loaded with biotinylated antigens to create a powerful antigen-specific CAR staining reagent (see CAR Dextramer® reagents). The dextran backbone also accommodates multiple fluorophores to generate a high signal intensity, and reagents can be designed with different fluorochromes. This opens the option of multiplexed staining to detect the presence of complex constructs, like bivalent or gated CARs.
Two other factors heighten the importance of using high-avidity reagents beyond the need to detect low-affinity CARs:
First, transduction efficiency varies widely depending on CAR construct. With low transduction rates, it is imperative to boost sensitivity so low-abundance CAR-engineered cells don’t go undetected (see our blog post “The proof is in the staining: a comparison of antigen-based CAR detection reagents”).
Second, expression rates can vary as well. When cells display fewer CARs, multivalent staining reagents, like those built on a dextran backbone, are more likely to bind them. CAR Dextramer® reagents outperform commonly used antibodies in antigen-specific detection of CAR-expressing cells. They show heightened sensitivity for low-expression CAR constructs, and they afford a combination of quality, performance consistency, and workflow ease unmatched by tetramer or monomer counterparts.
CAR Dextramer® reagents have one additional feature that makes them a superior choice for CAR cell detection and analysis: They can be combined with DNA barcodes (our dCODE® technology) to enable NGS-based discrimination of different population subsets or single-cell multi-omics, all from a single sample. You leave no cell undetected, and no cell unidentified.
So. What’s in your cytometer?
References
Related Resources
Cell Therapy
Explore how Dextramer® reagents support the development of effective cell therapies.
CAR-T Application Brochure
Learn how CAR Dextramer® technology enables superior detection and characterization of CAR cells.
Custom CAR Dextramer®
Our team can provide you with Custom CAR Dextramer® reagents to match your target antigen.
Interested in CAR Dextramer® Reagents for CAR Cell Detection?
Fill in the form and one of our dedicated specialists will get in touch with you shortly.

